Pharmacists don’t just fill prescriptions-they make critical decisions every day about which generic drugs are safe to substitute. But with over 90% of prescriptions filled with generics in the U.S., and new ones approved at a rate of nearly 100 per month, staying current isn’t optional. It’s a matter of patient safety.
Why Generics Knowledge Matters More Than Ever
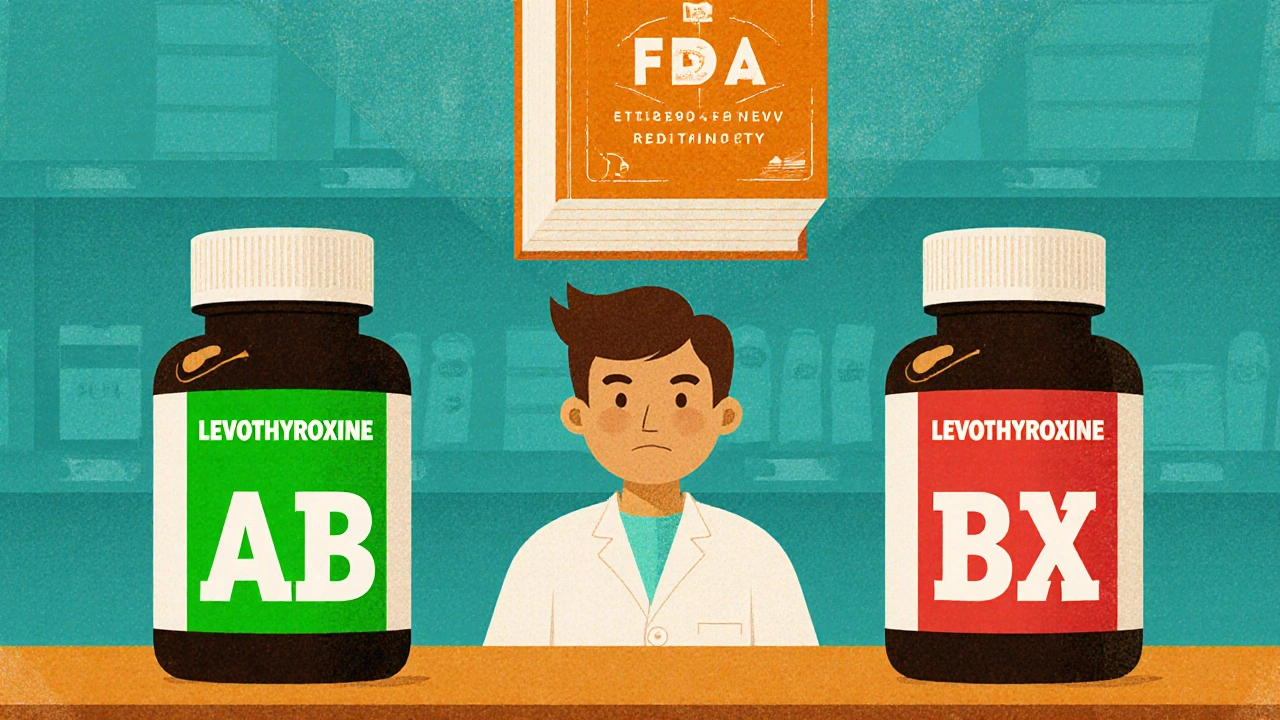
Generic drugs aren’t cheaper copies-they’re exact copies, legally required to match brand-name drugs in strength, purity, and performance. But that doesn’t mean they’re all interchangeable. The FDA’s Orange Book lists therapeutic equivalence ratings for over 1,200 generic products, and those ratings change monthly. A pharmacist who doesn’t track those updates could accidentally substitute a drug rated “AB” (therapeutically equivalent) with one rated “BX” (not recommended for substitution), especially with narrow therapeutic index drugs like levothyroxine or warfarin.
In 2021, 42.7% of all pharmacy malpractice claims involved errors tied to generic substitution. That’s not a small number. That’s nearly half of all errors linked to something pharmacists are trained to handle. And it’s not just about the science-it’s about the law. State laws vary wildly. Texas has strict rules on substituting biosimilars. New York requires written consent for certain generics. Illinois mandates cultural competency training alongside CE. One-size-fits-all courses won’t cut it.
What’s Required by Law
All 50 states require pharmacists to complete continuing education (CE) to renew their licenses. Most require between 15 and 30 hours every two years. But here’s the catch: not all of those hours count equally.
Only CE accredited by the Accreditation Council for Pharmacy Education (ACPE) or your state board counts. ACPE breaks CPE into categories. For generics, you need Category 2: Pharmacy Law and Regulations. That includes DEA number verification, controlled substance documentation, and state-specific substitution laws. In Illinois, you also need 1 hour each on sexual harassment prevention, implicit bias, and-starting January 2025-cultural competency. Missing one of those and your CE cycle is invalid.
Some states require proof of completion when you renew. California wants records kept for two years. New York demands certificates submitted with your renewal. Others, like Illinois, only ask if you’re audited. But if you’re licensed in multiple states? You’re juggling different rules, different deadlines, different topics. One CE course won’t cover them all.
What You Need to Know About Generic Drugs
Let’s get specific. The FDA requires generic drugs to show bioequivalence within 80-125% of the brand-name drug’s absorption rate. Sounds precise? It is. But it doesn’t mean all generics behave the same in every patient. Differences in inactive ingredients, manufacturing processes, or release mechanisms can affect absorption-especially in older adults or patients with gut conditions.
Then there’s the Abbreviated New Drug Application (ANDA) process. Generic manufacturers don’t repeat full clinical trials. Instead, they prove their drug performs like the original. But here’s a hidden hurdle: they must buy samples of the brand drug to test against. Under the CREATES Act, brand companies can’t block access. But in practice, delays still happen. That means new generics may take longer to hit the market than expected. Pharmacists need to know which ones are actually available and why.
Biosimilars add another layer. They’re not generics-they’re biologic drugs with complex structures. Interchangeability isn’t automatic. Only FDA-approved interchangeable biosimilars can be substituted without prescriber approval. And only a handful have that status so far. Yet 78% of hospital pharmacists say they need more training on them. If you’re working in a clinic or hospital, you can’t afford to guess.
How to Choose the Right CE Courses
Not all CE is created equal. Knowledge-based courses-where you watch a video and take a quiz-are the most common. But they’re also the least effective.
Pharmacists who complete application-based CE, like case studies or real-world scenarios, make 37% fewer substitution errors. Why? Because they’re forced to think like a pharmacist, not just memorize facts.
Look for courses that include:
- Case studies on levothyroxine substitutions
- Analysis of Orange Book rating changes
- State-specific substitution law summaries
- Interactive tools to check therapeutic equivalence ratings
Platforms like Pharmacist’s Letter and PocketPrep offer high-rated, application-based modules. PocketPrep alone has over 45,000 pharmacist users, and its generics content grew 32% last year. On CE21, application-based courses average 4.7 out of 5 stars. Knowledge-based ones? 3.2. The choice is clear.
Real Stories, Real Mistakes
Sarah Johnson, a community pharmacist in Ohio, shared a story on Reddit: “I almost substituted two different levothyroxine generics because they looked identical on the shelf. But I remembered a CE module that flagged the 2023 Orange Book change-only one was rated AB. I called the prescriber. The patient was on 100 mcg. The wrong one was 88 mcg. I caught it because of CE.”
That’s not rare. In 2023, a survey of 1,200 pharmacists found that 63% felt their CE courses were too generic-literally. They didn’t address state laws, didn’t explain why certain generics aren’t interchangeable, and didn’t prepare them for the next crisis.
One Texas pharmacist said: “I passed my CE, but when a patient asked why I couldn’t switch their generic, I had no answer. The course never mentioned Texas’s 2022 law requiring prescriber authorization for all narrow therapeutic index substitutions.”

What’s Changing in 2025
ACPE just announced new standards effective January 1, 2025. All generics-focused CE must now include:
- Training on biosimilar interchangeability
- Understanding FDA’s Risk Evaluation and Mitigation Strategies (REMS)
- Updates on the CREATES Act and its impact on drug access
That’s not a suggestion. That’s a requirement. If your CE provider hasn’t updated their curriculum by then, it won’t count.
NABP is also pushing for more consistency across states. Their goal? 80% alignment on generics education rules by 2025. That could mean less confusion for pharmacists who move or work in multiple states. But until then, you’re still responsible for knowing your state’s rules.
How to Stay on Top Without Burning Out
On average, pharmacists spend 27.5 hours a year on CE. But only 5.2 of those are dedicated to generics. That’s not enough. Experts say pharmacists with 10+ years of experience need 8-10 hours of targeted generics training annually. Newer pharmacists? 4-6 hours.
Here’s a simple plan:
- Check your state’s CE requirements by January each year.
- Identify 2-3 high-priority generics topics (e.g., biosimilars, narrow therapeutic index drugs).
- Choose one application-based course per quarter-don’t wait until the last month.
- Use free tools like the FDA Orange Book app to check ratings weekly.
- Join a local pharmacy group or online forum to discuss real cases.
Some hospital pharmacies now require extra training beyond state minimums. CVS Health piloted just-in-time learning tools at the point of sale. Pharmacists got pop-up alerts on their dispensing screens when a substitution risk was flagged. Result? 28% fewer errors. That’s the future-and it’s not far off.
Final Thought: CE Is Your Shield
Generics are everywhere. They’re cheaper, they’re legal, and they’re essential to keeping healthcare affordable. But they’re also complex, dynamic, and full of traps for the unprepared.
Continuing education isn’t about checking a box. It’s about protecting patients, protecting your license, and protecting your reputation. The best CE doesn’t just teach you what to do-it shows you why it matters. And in pharmacy, that’s the difference between a good pharmacist and a great one.
Do all states require the same continuing education for pharmacists?
No. Every state sets its own CE requirements for license renewal. While most require 15-30 hours every two years, the topics vary. Some states mandate training on sexual harassment, implicit bias, cultural competency, opioid alternatives, or biosimilars. Illinois requires 30 hours total, including three specific 1-hour topics. Texas has unique rules for narrow therapeutic index drugs. Always check your state board’s website before signing up for courses.
What’s the difference between a generic and a biosimilar?
Generics are chemically identical copies of small-molecule brand-name drugs. Biosimilars are highly similar versions of complex biologic drugs, like those used for cancer or autoimmune diseases. Unlike generics, biosimilars aren’t exact copies because biologics are made from living cells. Only FDA-approved interchangeable biosimilars can be substituted without prescriber permission-and very few have that status so far. Pharmacists need special training to understand the difference and when substitution is allowed.
How often is the FDA Orange Book updated?
The FDA updates the Orange Book monthly. New generic drugs are added, therapeutic equivalence ratings change, and patents expire or are challenged. Pharmacists should check the database regularly-especially before making substitutions. Many use the free FDA Orange Book app to get real-time updates. Relying on outdated information is one of the most common causes of substitution errors.
Can I use free CE courses to meet my requirements?
Yes, as long as they’re accredited by ACPE or your state board. Many reputable providers, like Pharmacist’s Letter, offer free ACPE-accredited modules on generics, ethics, and law. These are just as valid as paid courses. The key is accreditation-not cost. Always verify the provider’s ACPE provider number before completing the course.
What happens if I don’t complete my continuing education?
If you don’t complete your required CE hours by your renewal deadline, your license will expire. Most states allow a grace period of 30-90 days to complete missing hours, but you can’t practice during that time. After the grace period, you’ll need to reapply for licensure, pay late fees, and possibly retake exams. In extreme cases, you could face disciplinary action from your state board. Don’t risk your career-plan ahead.

Margo Utomo
YASSS, this post is literally the gospel we all need but never get 😍
Just last week I caught a BX-rated levothyroxine swap because I remembered that CE module from last quarter. My patient cried. I cried. We both lived. Thank you for reminding us that CE isn’t busywork-it’s a lifeline. 🙌💊